State the 3 sub-atomic particles and their relative charge and mass.

Tap here to flip back!
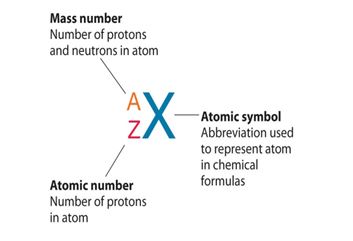
State how an atom of an element can be represented and what each symbol represents.
Tap here to flip back!
Define isotopes and state the similarities & differences between isotopes.
- Isotopes are atoms of the same element with the same no. of protons but different no. of neutrons.
- Isotopes have similar chemical properties (undergo same reactions) but different physical properties (mp/ bp).
Tap here to flip back!
State the max. no of electrons that each shell can accomodate; hence, deduce the electronic configuation of Ca atom (20 electrons).
1st shell = 2e;
2nd & 3rd shell = 8e
Ca: 2.8.8.2
Tap here to flip back!